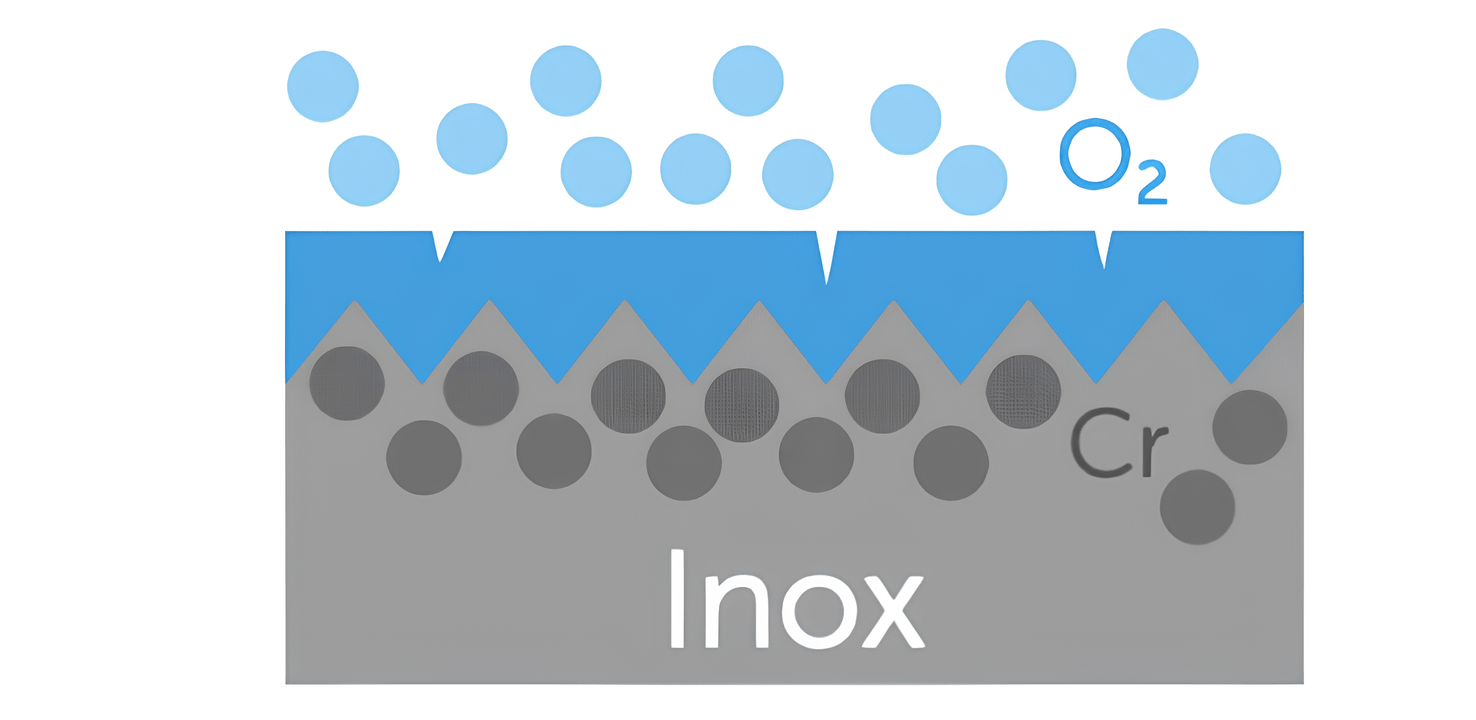
Passivation is a process by which a protective oxide film forms on the surface of a metal, reducing its rate of corrosion.
In the case of stainless steel, passivation results from the reaction between the chromium atoms present in the alloy and the oxygen in the air, leading to the formation of a chromium oxide layer. This layer acts as a protective barrier, significantly reducing environmental corrosion of the steel.
Passivation is influenced by several factors, including the electrochemical potential and pH of the medium. These factors determine the stability of the oxide formed.
PASSIVATION STAGES
In an aqueous environment, the formation of the passive film depends on a number of conditions. To ensure effective passivation, it is crucial that the metal surface is clean and free from contaminants that could prevent the formation of the oxide film.
Steps to achieve this include :
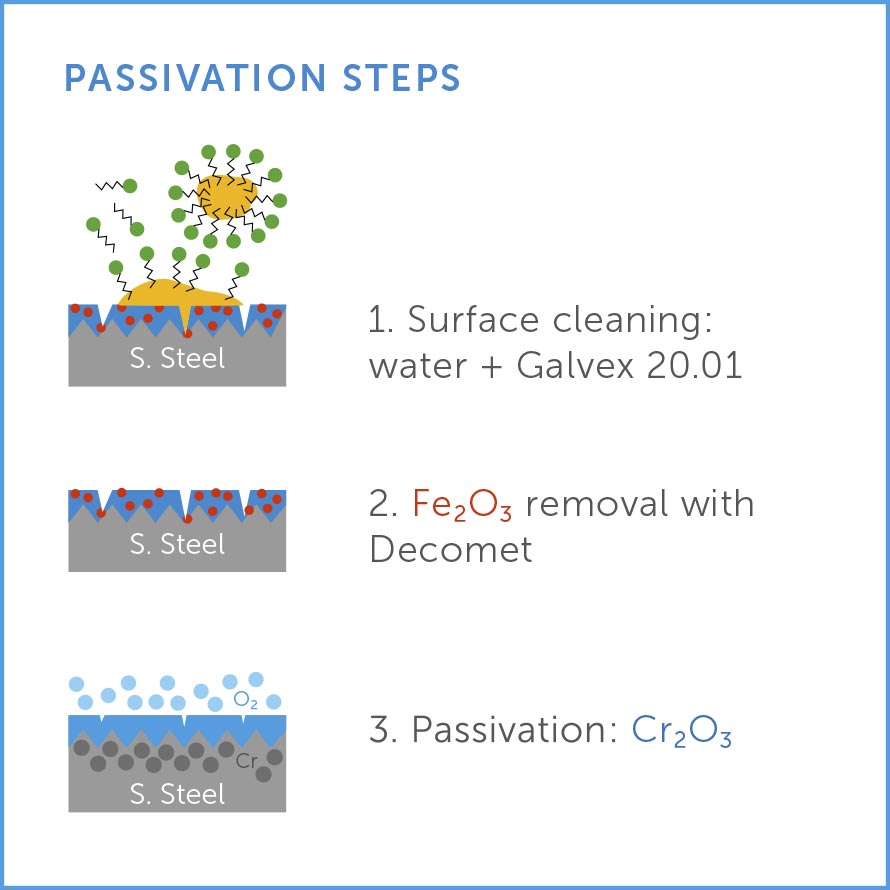
- Pickling of the part at the end of the manufacturing process to remove any obstacles to the formation of the oxide film. This process includes the removal of oils, greases and carbon steel particles (Galvex 20.01).
- Removal of thick oxide layers formed during heat treatment or welding operations, such as scale or slag. (DECOMET)
- Use of a process to facilitate passivation, such as citric acid treatment for stainless steel. (DECOMET)
EXAMPLE OF A PASSIVATION PROCESS
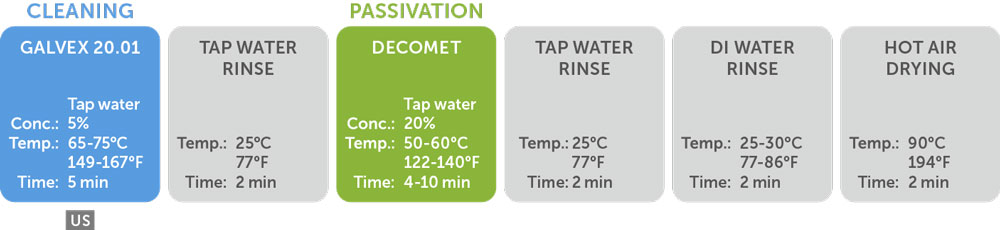
Here is an example of a complete passivation process proposed by NGL:
- Tap water cleaning with Galvex 20.01
- Tap water rinsing
- Tap water passivation with Decomet
- Tap water rinse x3
- Hot air drying
WHY USE PASSIVATION?
Passivation contributes to metal immunity, a condition in which the metal becomes stable and resistant to corrosion in a specific environment. This phenomenon is of vital importance in many industrial fields, as it improves the durability and performance of metals in corrosive environments.

7 ADVANTAGES OF USING CITRIC ACID FOR PASSIVATION
Citric acid passivation offers many advantages:
- Ecological and biodegradable: Unlike nitric acid, citric acid is a natural, biodegradable compound. This characteristic makes it more respectful of the environment, a crucial aspect in contemporary sustainability-oriented industrial practices such as NGL’s.
- Safe handling: Citric acid requires no special handling equipment, simplifying safety procedures and reducing costs associated with personal protective equipment. Its less corrosive nature compared to nitric acid makes it safer for operators.
- No harmful fumes: During the passivation process, citric acid does not emit nitrogen oxide fumes, which are not only harmful to operators but also to the environment.
- Chemical selectivity: Citric acid specifically targets free iron on the alloy surface, without affecting other constituent elements. This selectivity ensures effective removal of contaminants without compromising the alloy’s integral composition.
- Corrosion elimination efficiency: Compared with nitric acid, less citric acid is needed to achieve the same passivation results. This higher corrosion-elimination efficiency makes it an economical choice.
- Cleaning and polishing capabilities: In addition to its main function of passivation, citric acid has cleaning and polishing properties. It can therefore improve the aesthetic appearance of metal while protecting it from corrosion.
- Compliance with industry standards: The use of citric acid in passivation complies with ASTM A-380 and ASTM A-967 standards, ensuring that passivation processes meet industry quality and safety requirements.
SAFETY AND STANDARDS IN THE PASSIVATION PROCESS
Passivation requires compliance with strict safety standards and standardized procedures to ensure efficiency and safety.
ASTM standards A-380 and A-967 dictate precise guidelines for passivation procedures, including surface preparation, choice of acids, treatment conditions, and neutralization and rinsing methods. They guarantee the quality and safety of the process.
The handling of acids, such as nitric or citric acid, requires specific safety measures. Personnel must be trained and protected by appropriate equipment to prevent chemical hazards.
It is crucial to control exposure to vapors and chemicals, particularly with nitric acid, and to maintain adequate ventilation in work areas.
The passivation process involves meticulous cleaning of metal surfaces and controlled immersion in an acid solution. After passivation, it is important to neutralize and rinse metal parts to remove acid residues.
Post-passivation tests are necessary to confirm the effectiveness of the process and guarantee the reliability of the results.
Environmental considerations are important, with a preference for passivation products free from harmful compounds such as hexavalent chromium. Personnel safety is paramount, requiring adequate training in risks and emergency procedures.






